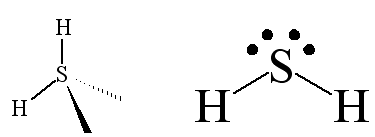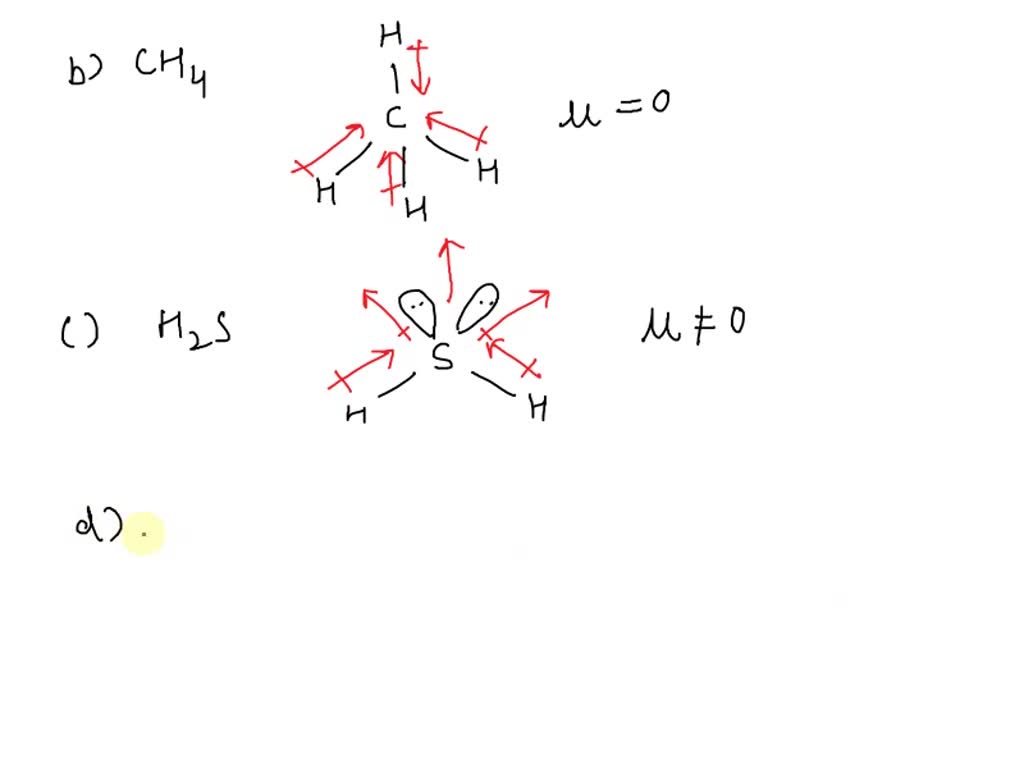
SOLVED: Which of the following species has the largest dipole moment (i.e., is the most polar)? a. H2 b. CH4 c. H2S d. H2O f. H2Se

Question The geometry of H2S and its dipole moment are: A. angular and non-zero B. angular and zero C. linear and non-zero D. linear and zero

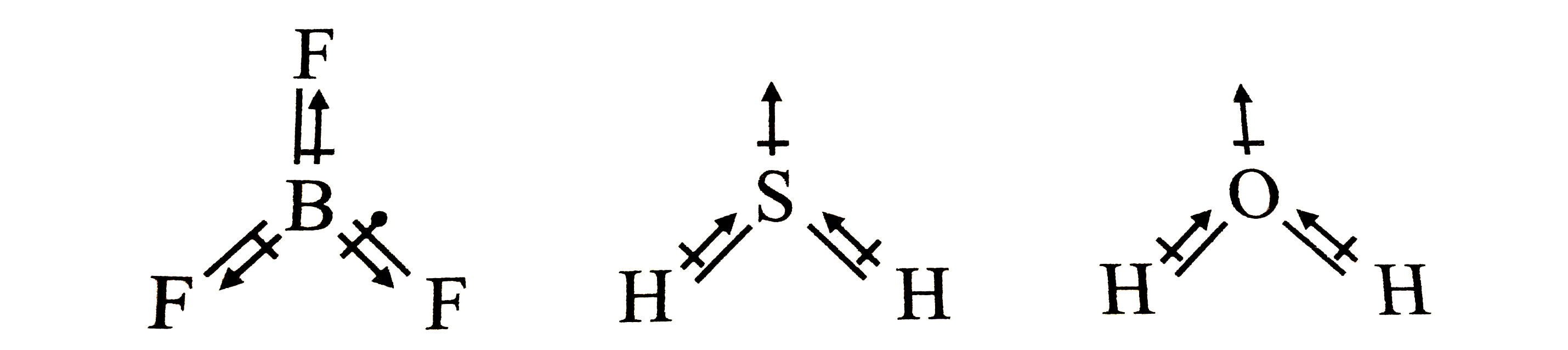
Arrange the following in order of increasing dipole moment H20,H2S,BF3 - Chemistry - Chemical Bonding and Molecular Structure - 12928599 | Meritnation.com

90 DUT8U J U TU Dipole moment of H2S is 0.95 D. Calculate the bond moment the bond angle is 97" (Cos 48.5° = 0.662) 0.7170 thi 1 275 Å The percentage of one

How to Calculate the Strength of Intermolecular Forces between Different Molecules from Chemical Structure | Chemistry | Study.com

out of Co2 and H2S which pair has dipole moment - Chemistry - Chemical Bonding and Molecular Structure - 12896827 | Meritnation.com


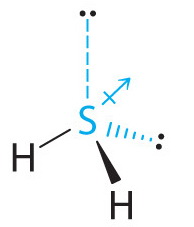







![PDF] The dipole moment surface for hydrogen sulfide H2S | Semantic Scholar PDF] The dipole moment surface for hydrogen sulfide H2S | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0ffa91c8852350858908b29f68b54676d71c7906/5-Table2-1.png)