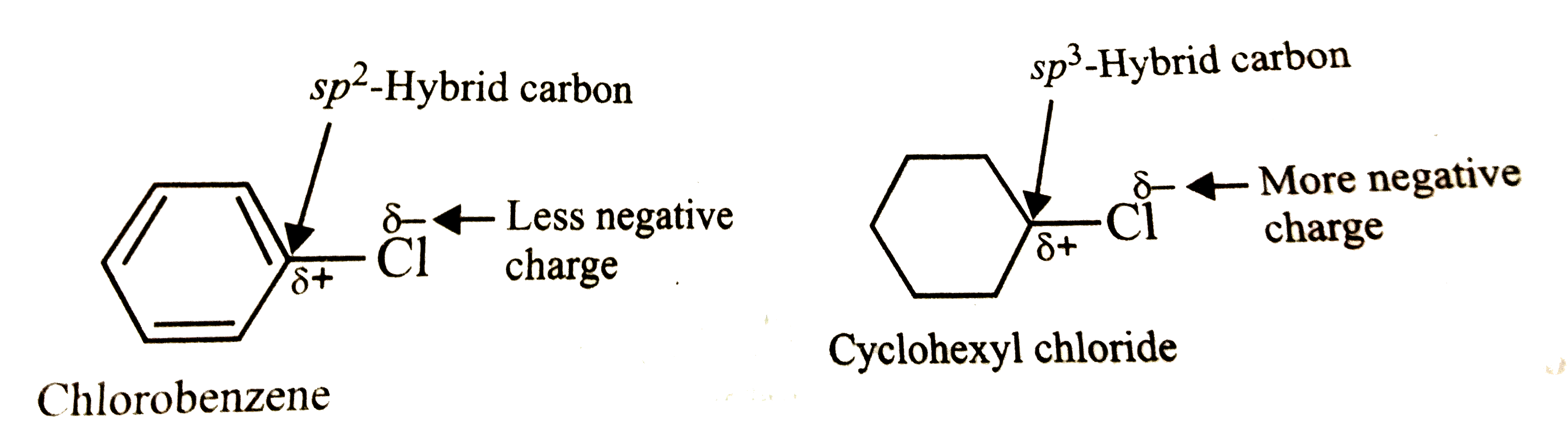
Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (i... - YouTube
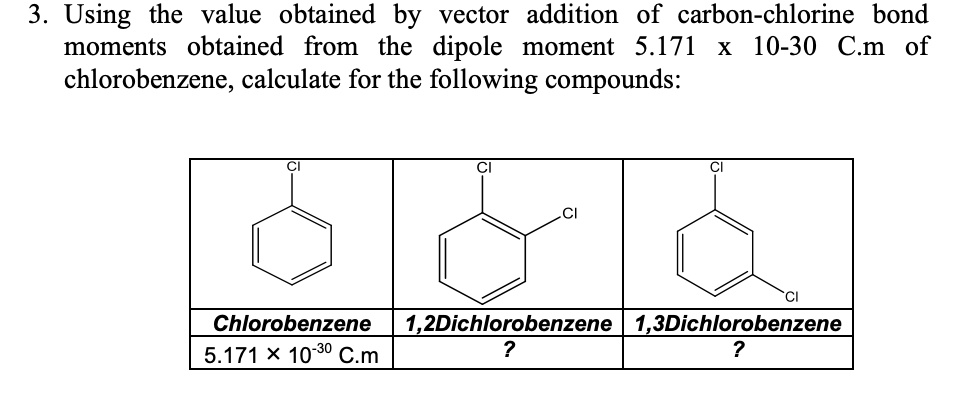
SOLVED: Using the value obtained by vector addition of carbon-chlorine bond moments obtained from the dipole moment 5.171 × 10^-30 Cm of chlorobenzene, calculate for the following compounds: Chlorobenzene, 1,2-Dichlorobenzene, 1,3-Dichlorobenzene, 5.171

Determine if the given species has a permanent dipole moment. Chlorobenzene, C6H5Cl | Homework.Study.com
Explain why: (a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? - Sarthaks eConnect | Largest Online Education Community

Arrange the following compounds in order of increasing dipole moments:I. ChlorobenzeneII. m dichlorobenzeneIII. o dichlorobenzeneIV. p dichlorobenzene

The dipole moment of chlorobenzene is 1.5D . Calculate dipole moment of 1,2,3,5 - tetrachlorobenzene.
e dipole moment of chlorobenzene is 1.5 D. Theighest dipole moment will be shown by(1) 1, 2 dichlorobenzene (2) 1, 2, 3 trichlorobenzene(3) 1, 2, 3, 4 tetrachlorobenzene(4) 1, 2, 3, 4, 5 pentacobenzene

The experimentally observed dipole moments for chlorobenzene, toluene, and 4-chlorotoluene are given below. Based on these values, explain whether the methyl group acts as an electron withdrawing or electron releasing group. Comment

Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (i... - YouTube
Explain why 1. The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? - Sarthaks eConnect | Largest Online Education Community
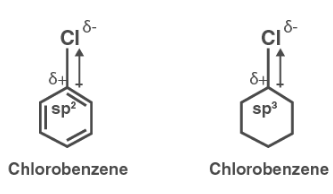
Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous

The dipole moment of chlorobenzene is `1.6D`m The expected dipole moment of meta-dichlorobenzene is: - YouTube