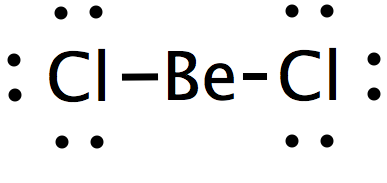How to calculate the hybridization state in the given formula of compund. Ex: The hybridization of BeCl2 in solid state and above 1200K is respectively (1) sp3, sp3 (2) sp3, sp2 (3)
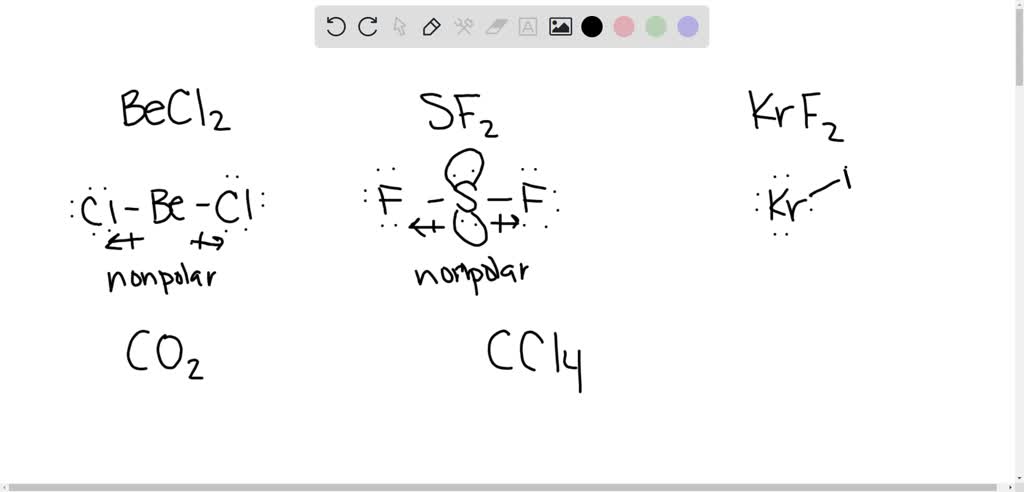
SOLVED: Which of the following molecules has a net dipole moment (which one is polar)? A) BeCl2 B) SF2 C) KrF2 D) CO2 E) CCl4

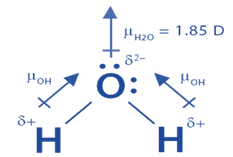
Question 17 Give reason the following: @ Dipole moment of BF3 is zero but ammonia has a dipole moment. (6) Cuci is covalent than Naci. C) LiCl is covalent than NaCl. (

Be-Cl is polar but Becl2 is not why - Chemistry - Chemical Bonding and Molecular Structure - 9585317 | Meritnation.com

Predict whether the dipole moment of dibromomethane molecules is zero, close to zero, or significantly greater than zero. | Homework.Study.com


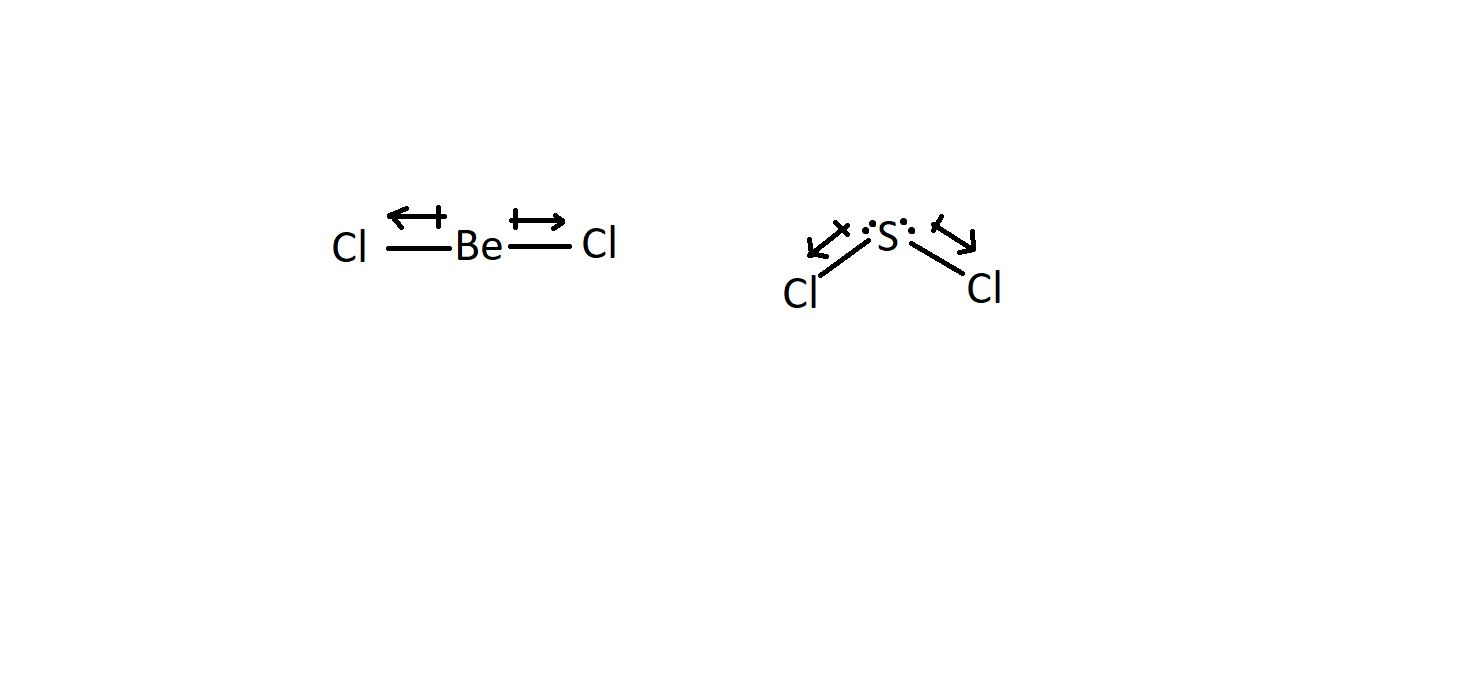




![Best Overview: Is BeCl2 Polar or Nonpolar [No#1] - Science Education and Tutorials Best Overview: Is BeCl2 Polar or Nonpolar [No#1] - Science Education and Tutorials](https://sciedutut.com/wp-content/uploads/2021/05/Is-BeCl2-Polar-or-Non-Polar-2-1024x493.png)